
- 430 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This book details chiroptical spectroscopic methods: electronic circular dichroism (ECD), optical rotatory dispersion (ORD), vibrational circular dichroism (VCD), and vibrational Raman optical activity (VROA). For each technique, the text presents experimental methods for measurements and theoretical methods for analyzing the experimental data. It also includes a set of experiments that can be adopted for undergraduate teaching laboratories. Each chapter is written in an easy-to-follow format for novice readers, with necessary theoretical formalism in appendices for advanced readers.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Polarized Light
Interaction between electromagnetic radiation and matter is the basis of a broad research area that is generally categorized as spectroscopy. When the matter under consideration is made up of chiral molecules (see Chapter 2), a special branch of spectroscopy, labeled as chiroptical spectroscopy, becomes pertinent. The words “chiroptical spectroscopy” can be construed to mean the study of chiral systems using optical spectroscopy methods. To understand the principles of chiroptical spectroscopy, it is necessary to gain insight into the properties of electromagnetic radiation, chiral molecules, and the interaction between them. These concepts form the basis for the starting chapters in this book.
1.1 Introduction to Light
Visible light represents electromagnetic radiation that can be detected with the human eye. This statement automatically raises thoughts about “invisible” light. To distinguish between visible and invisible light, we need to consider the wave properties of electromagnetic radiation. A wave (see Figure 1.1a) is associated with periodic oscillatory variation of its height (referred to as amplitude) in time. Two identical points in a wave constitute one cycle. For example, two consecutive crest points, two consecutive trough points, or three consecutive zero points constitute one cycle or one wave. If an observer stands at one point and watches the waves pass by in time, the number of cycles passed in a given second is labeled as frequency (ν). The unit of frequency is cycles/second (or simply s−1), which is designated as hertz (Hz; 1 Hz = s−1). A cosine wave with unit amplitude is represented by the function cos2πν t, where t stands for time in seconds. This cosine wave is shown in Figure 1.1a, where the definition of a cycle is depicted. The alternate representations of frequency of oscillation are wavelength and wavenumber. If a stationary wave is held along the z axis (see Figure 1.1b) and the distance between three consecutive zero points (or between two crests or between two troughs) of that wave is measured, then that distance is labeled as wavelength and represented by symbol λ [expressed as meters/cycle or simply as meters (m)]. This definition of wavelength is shown in Figure 1.1b, where a wave with unit amplitude, represented by the cosine function cos2πz/λ, is depicted. The product of wavelength and frequency of a wave is equal to the speed of that wave c, which is expressed as m·s−1:
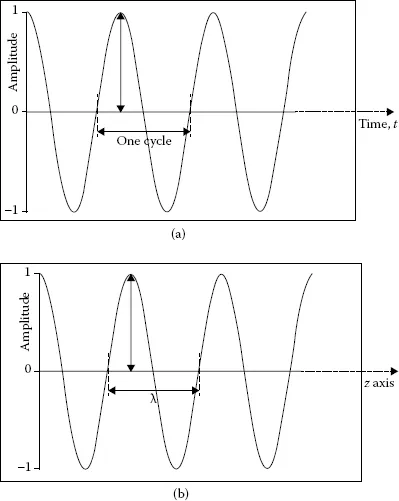
FIGURE 1.1
(a) Depiction of the amplitude and cycle of an oscillating cosine wave, cos 2πνt, with unit amplitude as a function of time. (b) Depiction of the amplitude and wavelength λ of an oscillating cosine wave, cos (2πz/λ), propagating along the z axis with unit amplitude.
(1.1) |
If an electromagnetic wave passes through a medium with refractive index nm, then the speed of electromagnetic wave changes as cm = c/nm. Then, Equation 1.1 modifies as cm = λmν, where λm is the wavelength of the wave in that medium (Levine 2009). For the current purposes, consider the waves propagating in vacuum, where nm = 1. Just as the number of cycles per second is labeled as frequency, the number of waves per unit length is labeled as wavenumber (). Since one wave occupies a length of λ, wavenumber is equal to the reciprocal of wavelength. Therefore, = 1/λ, and its units are expressed in m−1. Thus, an alternate form of Equation 1.1 is as follows:
(1.2) |
The wavelength λ (or equivalently frequency ν or wavenumber ) of a wave determines the properties of that wave. Human eyes respond to the electromagnetic waves of wavelength between ~400 and 800 nm (1 nm = 10−9 m), and this portion of electromagnetic radiation is referred to as the visible light. The different colors that human eyes can recognize (violet, indigo, blue, green, yellow, orange, and red, abbreviated as VIBGYOR) are the components of the visible light. The portions of electromagnetic radiation with wavelengths greater or less than the wavelengths in the visible light range are invisible to the human eye.
From here onward, the word “light” will be used to represent electromagnetic radiation (regardless of whether it is visible or invisible to the human eyes). The speed of light in vacuum is a constant, 2.99792458 × 108 m·s−1. Wavelength and frequency of a light wave are related through Equations 1.1 and 1.2. The different wavelength ranges of electromagnetic radiation constitute different portions of the electromagnetic “spectrum.” Selected portions of electromagnetic spectrum and their names are summarized in Table 1.1.
Wavelength is expressed in nanometers (nm) for the ultraviolet–visible region and micrometers (μm) for the infrared region. Wavenumber is usually expressed in cm−1. For example, a light wave with 200-nm wavelength represents ultraviolet light and corresponds to a wavenumber of 50,000 cm−1 and a frequency of 1.5 × 1015 Hz .
TABLE 1.1
Selected Regions of E...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Preface
- Author
- 1. Polarized Light
- 2. Chiral Molecules
- 3. Interaction of Circularly Polarized Light with Chiral Molecules
- 4. Quantum Chemical Formulation of Chiroptical Spectroscopy
- 5. Prediction of Chiroptical Spectra
- 6. Conformational Analysis
- 7. Experimental Methods for Measuring Chiroptical Spectra
- 8. Comparison of Experimental and Calculated Spectra
- 9. Chiral Aggregation, Equilibria, and the Horeau Effect
- 10. Applications of Chiroptical Spectra
- Appendixes
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Chiroptical Spectroscopy by Prasad L. Polavarapu in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Biochemistry in Medicine. We have over one million books available in our catalogue for you to explore.