
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
High Performance Polymers and Engineering Plastics
About this book
This book describes advances in synthesis, processing, and technology of environmentally friendly polymers generated from renewable resources. With contents based on a wide range of functional monomers and contributions from eminent researchers, this volume demonstrates the design, synthesis, properties and applications of plant oil based polymers, presenting an elaborate review of acid mediated polymerization techniques for the generation of green polymers. Chemical engineers are provided with state-of-the-art information that acts to further progress research in this direction.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
High Performance Polymers: An Overview
Abstract
During the last years, several new families of high performance polymers and engineering plastics have been reported which find enhanced application potential in the more challenging areas like aerospace, defense, energy, electronics, automotives etc. as compared to the commodity or conventional polymers. Such polymers provide improved set of properties like higher service-temperatures at extreme conditions and good mechanical strength, dimensional stability, thermal degradation resistance, environmental stability, gas barrier, solvent resistance, electrical properties etc. even at elevated temperatures.
Keywords: Poly(ether amide) and poly(ether amide-imide), poly(arylene ether), benzoxazine polymers, poly(ether ether ketone) (PEEK), polytriazole, hyperbranched conjugated polymers, alternating copolymers
1.1 Introduction
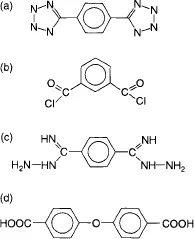
During the last years, several new families of high performance polymers and engineering plastics have been reported which find enhanced application potential in the more challenging application areas like aerospace, defense, energy, electronics, automotives etc. as compared to the commodity or conventional polymers. Such polymers provide improved set of properties like higher service-temperatures at extreme conditions and good mechanical strength, dimensional stability, thermal degradation resistance, environmental stability, gas barrier, solvent resistance, electrical properties etc. even at elevated temperatures. For example, aromatic polyesters and polybenzamide have decomposition temperatures around 480–500°C, whereas polybenzimidazole, polypyrrole and poly(p-phenylene) decompose around 650°C. Various other categories of high performance polymers include poly(phenylene ether), polysulfones, poly(aryl ether ketone), poly(oxadiazole), poly(imide), poly(ether amide), poly(ether amide imide), poly(naphthalene), liquid crystalline polymers and poly(amide imide) etc [1]. The raw materials involved in the synthesis of poly(phenylene ether) are described in Figure 1.1, whereas Figure 1.2 shows the chemical structures of the monomers used for the synthesis of poly(oxadiazole) polymers [1]. Poly(aryl ether ketone)s have aromatic groups in the main chain and both the ether group and the keto group are in the backbone. Liquid crystal polymers partly maintain the crystal structure is in the liquid phase above the melting point and exhibit a long range orientational order. Molecular structure/processability/property relationships of many of high performance polymers and engineering plastics have been reported in the literature along with their applications, a brief overview of a few of which is provided in the following sections.
Figure 1.1 Monomers used for the synthesis of poly(phenylene ether) [1], (a) 2,6-xylenol, (b) 2,3,6-trimethylphenol, (c) tetramethyldiphenylquinone and (d) 4-bromo-4′,4″-dihydroxytriphenylmethane.
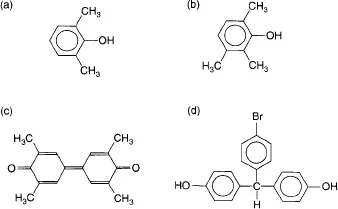
Figure 1.2 Monomers used for the synthesis of poly(oxadiazole) [1], (a) 1,4-phenylene-5,5’-tetrazole, (b) isophthaloyl chloride, (c) 1,4-benzenedicarboximidic acid dihydrazide and (d) 4,4’-diphenylether dicarboxylic acid.
1.2 Poly(ether amide) and Poly(ether amide-imide)
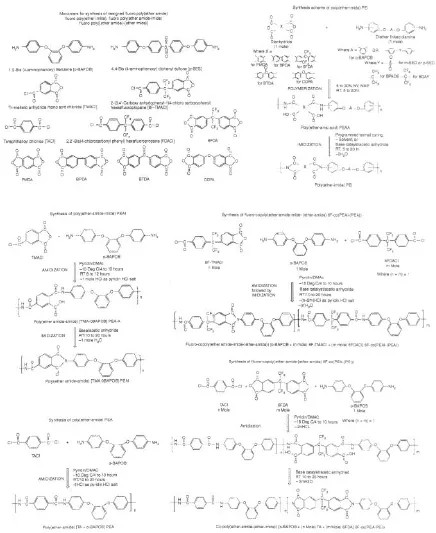
Vora [2] reported the synthesis and properties of high-performance thermoplastic fluoro-poly(ether amide)s (6F-PEA), fluoro-poly(ether amide-imide)s (6F-PEAI), and their co-polymers. The synthesis was based on the 6F-polyimide chemistry using using the novel state-of-the-art 2-(3,4’-carboxy anhydrophenyl-2(4-carboxyphenyl) hexafluoropropane (6F-TMA) and 2,2’-bis(4-carboxyphenyl) hexafluoropropane (6F-DAc) monomers. Various co-polymers like fluoro-copoly(ether amide-(ether imide))s (6F-co(PEA-PEI)), fluoro-copoly(ether amide-(ether amide-imide))s (6F-co(PEA-PEAI)) and fluorocopoly(ether amide-imide-(ether imide))s (6F-co(PEAI-PEI)) were also synthesized. The authors synthesized the films of the polymers and studied their their solution properties, solubility, morphology, thermal and thermo-oxidative stability, and moisture absorption. The polymers had high viscosity and high degree of polymerization with narrow polydispersity between 1.7 and 2.9. Figure 1.3 shows the synthesis schemes for these polymers and copolymers. It was ascertained by XRD spectroscopy that the polymers were amorphous in nature as no peaks were observed in the diifractograms measured in the range of 10 to 35° 2θ. The polymers were observed to be soluble in almost all organic solvents and the films prepared by thermal curing at elevated temperature were either partially soluble or insoluble in such solvents indicating increased solvent resistance. The polymers were observed to possess moderate to high glass transition temperatures (Tg). The TGA analysis indicated that the 5% weight losses for the polymers in air were in the range of 480–515°C. The weight loss in nitrogen was observed on an average about 15–25°C higher than in air. The polymers also had excellent thermal resistance in isothermal heating at temperature 300°C for 300 h. Most of the polymers had low moisture uptake at 100% relative humidity at 50°C over 100 h. The amorphous nature of the polymers led to their easy processability into films, sheets, molded articles, etc. Dielectric constant values of all the synthesized fluorinated polymers was observed in the range of 2.85 to 3.1 measured at 1 kHz at 25°C. These values were also lower than the values reported for the commercially available non-fluorinated polymers. The authors also commented on the enhancement of polymer properties by the addition of inorganic montmorillonite clays as filler.
Figure 1.3 Chemical structures of the monomers, poly(ether amide) & poly(ether amide-imide) polymers and their copolymers. Reproduced from reference 2 with permission from Elsevier.
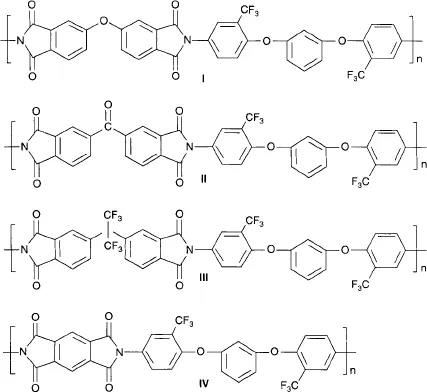
Xie et al. [3] also reported the synthesis of polyimides with low moisture absorption and high hygrothermal stability. Four different aromatic dianhydrides, viz. 4,4’-oxydiphthalic anhydride (ODPA), 3,3’,4,4’-benzophenone tetracarboxylic dianhydride, 4,4’-(hexafluoroisopropylidene)diphthalic anhydride, and pyromellitic dianhydride were used during the synthesis and the resulting polyimides are shown respectively in Figure 1.4. The authors observed better solubility over a wider range of solvents in the case of chemically imidized films when compared to the films prepared by thermal methods owing to more compact structure due to stronger aggregation of the polyimide molecules during thermal imidization. The authors also suggested that the cemical method leads to incomplete imidization which was the cause of lower stability of the films generated by this method.
Figure 1.4 Structures of poly(ether imide) polymers synthesized from 1,3-bis (4-amino-2-trifluoromethylphenoxy)benzene and various anhydrides. Reproduced from reference 3 with permission from Elsevier.
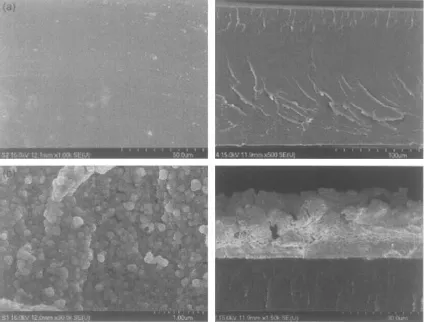
Rajagopalan et al. [4] reported the synthesis of sulphonated polyetherimide and subsequent synthesis of ionic polymer metal composites (IPMC) by depositing platinum on both sides of the polymer membrane by electroless plating process for use in actuators. The TGA and NMR analysis confirmed the successful incorporation of sulfonic groups in the polymer backbone. The content of sulfur in the polymer membrane was measured to be 4.68% by EDX analysis and the degree of sulfonation could also be controlled. SEM micrographs of the composite membrane also confirmed the uniform formation of small platinum particles on the surface of polymer membrane as shown in Figure 1.5. The thickness of platinum coating was observed to be 15–18 μm. The surface of the uncoated membrane was very smooth whereas platinum deposition led to the formation of rough surface morphology. The ionic polymer–metal composite actuator showed good harmonic and step responses similar to an electro-active polymer.
Figure 1.5 SEM micrographs of the (a) surface and cross-section of the sulfonated PEI membrane and (b) surface and cross-section of the platinum coated sulfonated PEI membrane. Reproduced from reference 4 with permission from Elsevier.
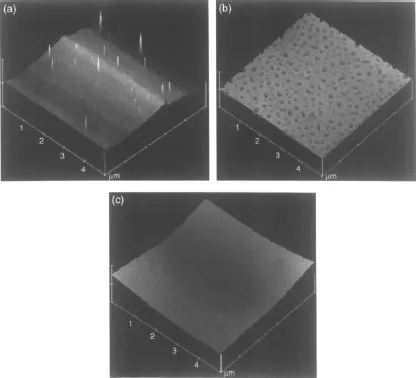
Guhathakurta et al. [5] characterized the polyelectrolytes based on sulfonated PEI and triazole. Bisphenol A based polyetherimide was sulfonated using trimethylsilylchlorosulfonate (TMSCS) as sulfonating agent. Polyelectrlayes were prepared by solution blending of sulfonated PEI and triazole in the presence of dimethylacet-amide. The amount of sulfonated PEI and traizole was altered, the PEI had also different degrees of sulfonation. The effect of degree of sulfonation in the sulfonated PEI and triazole concentration in the blend on size, shape and crystal morphology of triazole crystals in sulfonated polyetherimide were examined. It was observed that at a constant triazole weight percent, increased sulfonation level caused enhanced nucleation density, reduction of crystallite size and their uniform distribution throughout the polymer matrix as shown in Figure 1.6. The crystal domains were also elevated at lower sulfonation level and embedded at higher level of sulfonation.
Figure 1.6 Tapping mode three dimensional topographic images of suphonated PEI and triazole (70:30) polyelectrolytes. Degree of suphonation (a) 22%, (b) 48% and (c) 62%. Reproduced from reference 5 with permission from Elsevier.
1.3 Poly(arylene ether)
Dhara et al. [6] reviewed the synthesis and properties of poly(arylene ether) polymers. The role of trifluoromethyl groups on the polymerization process and polymer properties was also demonstrated. Kim et al. [7] also reported the synthesis of hyperbranched pol(arylene ether) polymer. The selective and sequential displacement of the fluorine group and the nitro group of 5-fluoro-2-nitrobenzotriflu-oride was used as a basis for the synthesis of th...
Table of contents
- Cover
- Half Title page
- Title page
- Copyright page
- Preface
- Contributors
- Chapter 1: High Performance Polymers: An Overview
- Chapter 2: Synthesis and Properties of Polyoxadiazoles
- Chapter 3: Conjugated Polymers Based on Benzo[1,2-b:4,5-b’]dithiophene for Organic Electronics
- Chapter 4: Polysulfone-Based Ionomers
- Chapter 5: High-Performance Processable Aromatic Polyamides
- Chapter 6: Phosphorus-Containing Polysulfones
- Chapter 7: Synthesis and Characterization of Novel Polyimides
- Chapter 8: The Effects of Structures on Properties of New Polytriazole Resins
- Chapter 9: High Performance Fibers
- Chapter 10: Synthesis and Characterization of Poly (aryl ether ketone) Copolymers
- Chapter 11: Liquid Crystalline Thermoset Epoxy Resins
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access High Performance Polymers and Engineering Plastics by Vikas Mittal in PDF and/or ePUB format, as well as other popular books in Technologie et ingénierie & Ingénierie de la chimie et de la biochimie. We have over one million books available in our catalogue for you to explore.