
eBook - ePub
Basic Pharmacokinetics and Pharmacodynamics
An Integrated Textbook and Computer Simulations
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Basic Pharmacokinetics and Pharmacodynamics
An Integrated Textbook and Computer Simulations
About this book
Updated with new chapters and topics, this book provides a comprehensive description of all essential topics in contemporary pharmacokinetics and pharmacodynamics. It also features interactive computer simulations for students to experiment and observe PK/PD models in action.
• Presents the essentials of pharmacokinetics and pharmacodynamics in a clear and progressive manner
• Helps students better appreciate important concepts and gain a greater understanding of the mechanism of action of drugs by reinforcing practical applications in both the book and the computer modules
• Features interactive computer simulations, available online through a companion website at: https://web.uri.edu/pharmacy/research/rosenbaum/sims/
• Adds new chapters on physiologically based pharmacokinetic models, predicting drug-drug interactions, and pharmacogenetics while also strengthening original chapters to better prepare students for more advanced applications
• Reviews of the 1st edition: "This is an ideal textbook for those starting out … and also for use as a reference book …." (International Society for the Study of Xenobiotics) and "I could recommend Rosenbaum's book for pharmacology students because it is written from a perspective of drug action . . . Overall, this is a well-written introduction to PK/PD …. " (British Toxicology Society Newsletter)
• Presents the essentials of pharmacokinetics and pharmacodynamics in a clear and progressive manner
• Helps students better appreciate important concepts and gain a greater understanding of the mechanism of action of drugs by reinforcing practical applications in both the book and the computer modules
• Features interactive computer simulations, available online through a companion website at: https://web.uri.edu/pharmacy/research/rosenbaum/sims/
• Adds new chapters on physiologically based pharmacokinetic models, predicting drug-drug interactions, and pharmacogenetics while also strengthening original chapters to better prepare students for more advanced applications
• Reviews of the 1st edition: "This is an ideal textbook for those starting out … and also for use as a reference book …." (International Society for the Study of Xenobiotics) and "I could recommend Rosenbaum's book for pharmacology students because it is written from a perspective of drug action . . . Overall, this is a well-written introduction to PK/PD …. " (British Toxicology Society Newsletter)
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Basic Pharmacokinetics and Pharmacodynamics by Sara E. Rosenbaum in PDF and/or ePUB format, as well as other popular books in Medicine & Biostatistics. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
Introduction to Pharmacokinetics and Pharmacodynamics
SARA E. ROSENBAUM
- 1.1 Introduction: Drugs and Doses
- 1.2 Introduction to Pharmacodynamics
- 1.2.1 Drug Effects at the Site of Action
- 1.2.1.1 Interaction of a Drug with Its Receptor
- 1.2.1.2 Postreceptor Events
- 1.2.2 Agonists, Antagonists, and Concentration–Response Relationships
- 1.2.1 Drug Effects at the Site of Action
- 1.3 Introduction to Pharmacokinetics
- 1.3.1 Plasma Concentration of Drugs
- 1.3.2 Processes in Pharmacokinetics
- 1.4 Dose–Response Relationships
- 1.5 Therapeutic Range
- 1.5.1 Determination of the Therapeutic Range
- 1.6 Summary
- Reference
Objectives
The material in this chapter will enable the reader to:
- Define pharmacodynamics and pharmacokinetics
- Understand the processes that control the dose–response relationship
- Gain a general appreciation of how mathematical expressions in pharmacodynamics and pharmacokinetics can be used for the rational determination of optimum dosing regimens
1.1 Introduction: Drugs and Doses
Drugs may be defined as chemicals that alter physiological or biochemical processes in the body in a manner that makes them useful in the treatment, prevention, or cure of diseases. Based on this definition, any useful drug must affect body physiology or biochemistry. By extension, any useful drug must, if used inappropriately, possess the ability to do harm. Drug action begins with administration of the drug (input) and concludes with the biological response (output, which can be a beneficial and/or an adverse effect). The inputs (dose, frequency of administration, and route of administration) must be selected carefully to optimize the onset, intensity, and duration of therapeutic effects for a particular disease condition. At the same time, the inputs selected must minimize any harmful effects of drugs.
The design of optimum dosing regimens requires a complete understanding of the processes and steps that translate the input into the output. It also requires an understanding of how the input–output relationship may be influenced by individual patient characteristics that may exist at the very beginning of therapy, as well as conditions that may arise during the course of drug therapy. These will include the age and weight of the patient, the presence of other diseases, genetic factors, concurrent medications, and changes in the disease being treated over time.
The material presented in this book will address and explain why, as shown in Table 1.1, there is such tremendous variability in the value of drug doses and dosing frequencies among therapeutic drugs. Additionally, it will address why different routes of administration are used for different drugs and different indications (Table 1.1).
Table 1.1 Examples of Common Daily Doses and Dosing Intervals
| Drug | Daily Dose (mg) | Dose Frequency (h) | Route |
| Calcium carbonate | 3000 | 2 | Oral |
| Ibuprofen | 1600 | 6 | Oral |
| Vancomycin (for MRSAa) | 2000 | 12 | Intravenous |
| Amoxicillin | 750 | 8 | Oral |
| Vancomycin (for pseudomembranous colitis) | 1000 | 6 | Oral |
| Atenolol | 100 | 24 | Oral |
| Fluoxetine | 20 | 24 | Oral |
| Ramipril | 10 | 12 | Oral |
| Digoxin | 0.250 | 24 | Oral |
| Chloroquine | 300 | Weekly | Oral |
aMethicillin-resistant Staphylococcus aureus.
The steps between drug input and the emergence of the response can be broken down into two phases: pharmacokinetic and pharmacodynamic. The pharmacokinetic phase encompasses all the events between the administration of a dose and the achievement of drug concentrations throughout the body. The pharmacodynamic phase encompasses all the events between the arrival of the drug at its site of action and the onset, magnitude, and duration of the biological response (Figure 1.1). The rational design of optimum dosing regimens must be based on a thorough understanding of these two phases and will, ideally, include the development of one or more mathematical expressions for the relationship between dose and the time course of drug response.
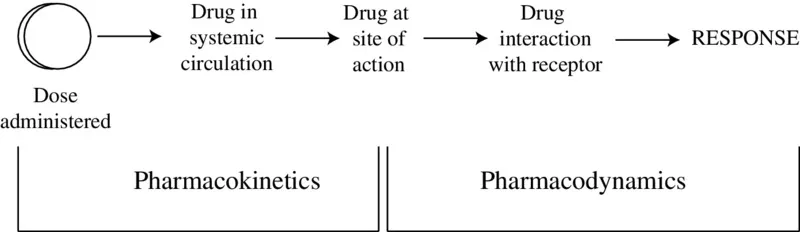
Figure 1.1 The two phases of drug action. The pharmacokinetic phase is concerned with the relationship between the value of the dose administered and the value of the drug concentrations achieved in the body; the pharmacodynamic phase is concerned with the relationship between drug concentrations at the site of action and the onset, intensity, and duration of drug response.
Optimum drug administration is important not only for ensuring good patient outcomes in clinical practice, but also in the design of clinical trials during drug development. The cost of drug research and development is enormous, so it is critical that all drug candidates selected for human trials are evaluated in the most efficient, cost-effective manner possible.
The application of pharmacokinetic and pharmacodynamic principles to this process has been shown to enhance the selection of optimum doses and optimum designs of phase II clinical trials.
1.2 Introduction to Pharmacodynamics
Pharmaco- comes from the Greek word for “drug,” pharmackon, and dynamics means “of or relating to variation of intensity.” Pharmacodynamics (PD) is the study of the magnitude of drug response. In particular, it is the study of the onset, intensity, and duration of drug response and how these are related to the concentration of a drug at its site of action. An overview of some basic drug terminology and the drug response–concentration relationship is provided below.
1.2.1 Drug Effects at the Site of Action
Note that although some references and textbooks distinguish the terms drug effect and drug response, this distinction has not been adopted universally. In this book, effect and response are used interchangeably.
1.2.1.1 Interac...
Table of contents
- Cover
- Title page
- Copyright
- Dedication
- Preface
- Contributors
- Chapter 1 Introduction to Pharmacokinetics and Pharmacodynamics
- Chapter 2 Passage of Drugs Through Membranes
- Chapter 3 Drug Administration and Drug Absorption
- Chapter 4 Drug Distribution
- Chapter 5 Drug Elimination and Clearance
- Chapter 6 Compartmental Models in Pharmacokinetics
- Chapter 7 Pharmacokinetics of an Intravenous Bolus Injection in a One-Compartment Model
- Chapter 8 Pharmacokinetics of an Intravenous Bolus Injection In A Two-Compartment Model
- Chapter 9 Pharmacokinetics of Extravascular Drug Administration
- Chapter 10 Introduction to Noncompartmental Analysis
- Chapter 11 Pharmacokinetics of Intravenous Infusion in a One-Compartment Model
- Chapter 12 Multiple Intravenous Bolus Injections in the One-Compartment Model
- Chapter 13 Multiple Intermittent Infusions
- Chapter 14 Multiple Oral Doses
- Chapter 15 Nonlinear Pharmacokinetics
- Chapter 16 Introduction to Pharmacogenetics
- Chapter 17 Models Used to Predict Drug–Drug Interactions for Orally Administered Drugs
- Chapter 18 Introduction to Physiologically Based Pharmacokinetic Modeling
- Chapter 19 Introduction to Pharmacodynamic Models and Integrated Pharmacokinetic–Pharmacodynamic Models
- Chapter 20 Semimechanistic Pharmacokinetic–Pharmacodynamic Models
- Appendix A Review of Exponents and Logarithms
- Appendix B Rates of Processes
- Appendix C Creation of Excel Worksheets for Pharmacokinetic Analysis
- Appendix D Derivation of Equations for Multiple Intravenous Bolus Injections
- Appendix E Enzyme Kinetics: Michaelis–Menten Equation and Models for Inhibitors and Inducers of Drug Metabolism
- Appendix F Summary of the Properties of the Fictitious Drugs used in the Text
- Appendix G Computer Simulation Models
- Glossary of Terms
- Index
- EULA