
eBook - ePub
Photosensitization of Porphyrins and Phthalocyanines
Ichiro Okura
This is a test
Compartir libro
- 252 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Photosensitization of Porphyrins and Phthalocyanines
Ichiro Okura
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
Photosensitization of Porphyrins and Phthalocyanines covers the scentific background to porphyrins and phthalocyanines, and applications of the compounds, especially for the application for photosensitization. It also has a review of advances in research and applications in this field.
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Photosensitization of Porphyrins and Phthalocyanines un PDF/ePUB en línea?
Sí, puedes acceder a Photosensitization of Porphyrins and Phthalocyanines de Ichiro Okura en formato PDF o ePUB, así como a otros libros populares de Medicina y Biochimica in medicina. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
Part I
Synthesis and Characterization of Porphyrins and Phthalocyanines
Chapter 1 Preparation of Porphyrins and Phthalocyanines
1.1 General Methods for Preparation of Porphyrins
Porphyrins are classified into three categories: (A) porphyrins prepared from natural heme, (B) alkyl-substituted porphyrins and (C) meso-tetraphenylporphyrin and its derivatives. In this section, the preparation methods of the representative porphyrins in each category are described. The preparation methods of water-soluble porphyrins (D), metalloporphyrins (E) and chlorin derivatives (F) are also provided.
1.1.1 Synthesis of porphyrins from natural heme (Scheme 1-1)
Protoporphyrin IX (1) is prepared from protohemin as a starting material. Iron ion in the protohemin is removed by refluxing with iron in formic acid. After demetallation, protoporphyrin IX is obtained by the addition of ammonium acetate. Protoporphyrin IX methylester (2) is synthesized by esterification of protoporphyrin IX in HCl-methanol solution.1)
Hematoporphyrin IX (3) is prepared as follows. Protoporphyrin IX is treated with HBr-acetic acid to convert the vinyl group to a hydroxyl group and is then neutralized by NaOH. By the addition of excess NaOH solution, hematoporphyrin IX is obtained as a precipitate.
meso-Porphyrin IX (4) dimethylester is prepared as follows.2) The vinyl group of hematoporphyrin IX dimethylester is reduced to an ethyl group by hydrogen gas catalyzed by palladium-active carbon under methylmethacryrate and formic acid. meso-Porphyrin IX dimethylester is easily recrystallized from chloroform-methanol. This compound is most popular among the porphyrins from natural heme.
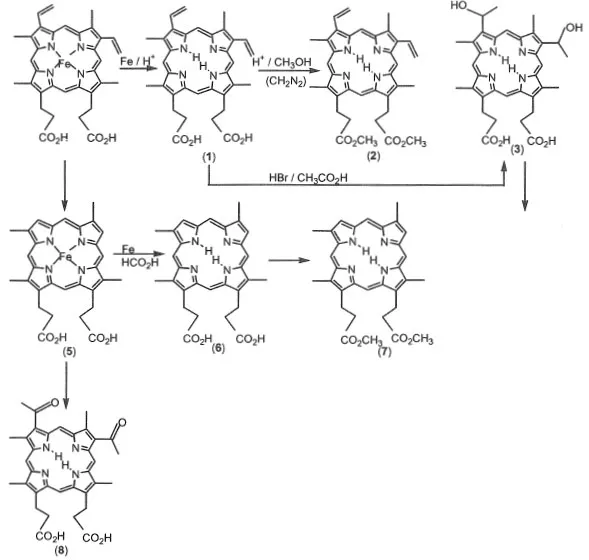
Scheme 1-1 Synthesis of porphyrins from natural heme.
Deuteroporphyrin IX (5) is prepared from protohemin as the starting material.3) Deuterohemin is produced by refluxing protohemin with resorcinol. Iron ion in deuterohemin is removed by refluxing with iron in formic acid. After demetallation, deuteroporphyrin IX methylester is obtained by esterification of protoporphyrin IX in HCl-methanol solution. 2,4-Diacetyldeuteroporphyrin IX is synthesized by the acylation of deuterohemin treated with acetic anhydride and tin chloride.3)
2,4-Diformyldeuteroporphyrin IX is prepared by the oxidation of protoporphyrin IX dimethylester using KMnO4/MgSO4 as oxidizing reagents in acetone. 2,4-Diformyldeuteroporphyrin IX is insoluble in organic solvents.4)
1.1.2 Synthesis of alkyl-substituted porphyrins
The representative alkyl-substituted porphyrins are etioporphyrin (EtioP) and octaethylporphyrin (OEP).5) There are three synthesis routes of OEP, as shown in Scheme 1-2.
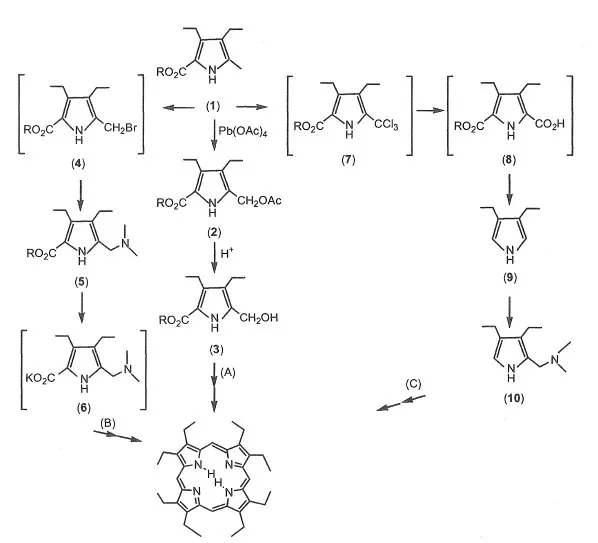
Scheme 1-2 Synthesis routes of OEP.
Route A5): The derivative (2) is synthesized by the acylation of a-methyl of pyrrole derivative (1) using Pb(OAc)4. The derivative (3) is prepared by hydrolysis of (2) in alkaline solution. OEP is synthesized by cyclization and oxidation of (3). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystallization from chloroform and methanol.
Route B6): The derivative (4) is synthesized by bromination of (1). The diethylamino-methyl derivative (5) is synthesized by nucleophilic substitution of amine to (4). The potassium salt (6) is obtained by hydrolysis of (5). OEP is synthesized by cyclization and oxidation of (6). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystal-lization from chloroform and methanol.
Route C7): 3,4-Diethylpyrrol (9) is prepared by decarboxylation of (8). The derivative (10) is synthesized by aminomethylation of (9) in formaldehyde and dimethylformamide. OEP is prepared by cyclization and oxidation of (10). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystallization from chloroform and methanol.
Octamethylporphyrin (OMP) is synthesized using the above methods.8) The yield of OMP is very low and OMP is insoluble in organic solvent.
1.1.3 Synthesis of meso-tetraphenylporphyrins
Rothemund synthesized meso-tetraphenylporphyrin(TPP) by condensation between pyrrole and benzaldehyde.9) Since this report, meso-tetraphenylporphyrin derivatives are synthesized using benzaldehyde derivatives. The yield of porphyrin improves especially when propionic acid is used as a solvent. Porphyrins are usually purified using column chromatography (silica gel or almina).10) The synthesis route is shown in Scheme 1-3. Tetranitrophenylporphyrin, tetratolylporphyrin, tetra(4-pyridyl) porphyrin and tetra(pentafluorophenyl)porphyrin are synthesized by this method.
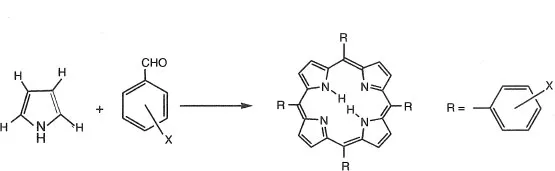
Scheme 1-3 Synthesis routes of meso-tetraphenylporphyrin derivatives.
1.1.4 Synthesis of water-soluble porphyrins
Water-soluble porphyrins, tetraphenylporphyrin tetrasulfonate (TPPS), is synthesized by the sulfonation of TPP using sulfuric acid. Tetrakis-(aminophenyl)porphyrin (TAPP) is synthesized by the reduction of tetrakis(4-nitrophenyl) porphyrin under Sn / HCl condition. Tetrakis-(4-carboxyphenyl)porphyrin (TCPP) is synthesized by hydrolysis of tetrakis-(4-methylcarboxyphenyl)-porphyrin in alkaline alcohol solution. Tetrakis-(4-methylpyridyl)porphyrin (TMPyP) is prepared by quaterization of tetra(4-pyridyl)porphyrin with methyliodide or p-toluenesulfonate methylester in DMF.
1.1.5 Synthesis of metalloporphyrins
Metalloporphyrins are widely use...