
eBook - ePub
Photosensitization of Porphyrins and Phthalocyanines
Ichiro Okura
This is a test
Partager le livre
- 252 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Photosensitization of Porphyrins and Phthalocyanines
Ichiro Okura
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Photosensitization of Porphyrins and Phthalocyanines covers the scentific background to porphyrins and phthalocyanines, and applications of the compounds, especially for the application for photosensitization. It also has a review of advances in research and applications in this field.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Photosensitization of Porphyrins and Phthalocyanines est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Photosensitization of Porphyrins and Phthalocyanines par Ichiro Okura en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Medicina et Biochimica in medicina. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
Part I
Synthesis and Characterization of Porphyrins and Phthalocyanines
Chapter 1 Preparation of Porphyrins and Phthalocyanines
1.1 General Methods for Preparation of Porphyrins
Porphyrins are classified into three categories: (A) porphyrins prepared from natural heme, (B) alkyl-substituted porphyrins and (C) meso-tetraphenylporphyrin and its derivatives. In this section, the preparation methods of the representative porphyrins in each category are described. The preparation methods of water-soluble porphyrins (D), metalloporphyrins (E) and chlorin derivatives (F) are also provided.
1.1.1 Synthesis of porphyrins from natural heme (Scheme 1-1)
Protoporphyrin IX (1) is prepared from protohemin as a starting material. Iron ion in the protohemin is removed by refluxing with iron in formic acid. After demetallation, protoporphyrin IX is obtained by the addition of ammonium acetate. Protoporphyrin IX methylester (2) is synthesized by esterification of protoporphyrin IX in HCl-methanol solution.1)
Hematoporphyrin IX (3) is prepared as follows. Protoporphyrin IX is treated with HBr-acetic acid to convert the vinyl group to a hydroxyl group and is then neutralized by NaOH. By the addition of excess NaOH solution, hematoporphyrin IX is obtained as a precipitate.
meso-Porphyrin IX (4) dimethylester is prepared as follows.2) The vinyl group of hematoporphyrin IX dimethylester is reduced to an ethyl group by hydrogen gas catalyzed by palladium-active carbon under methylmethacryrate and formic acid. meso-Porphyrin IX dimethylester is easily recrystallized from chloroform-methanol. This compound is most popular among the porphyrins from natural heme.
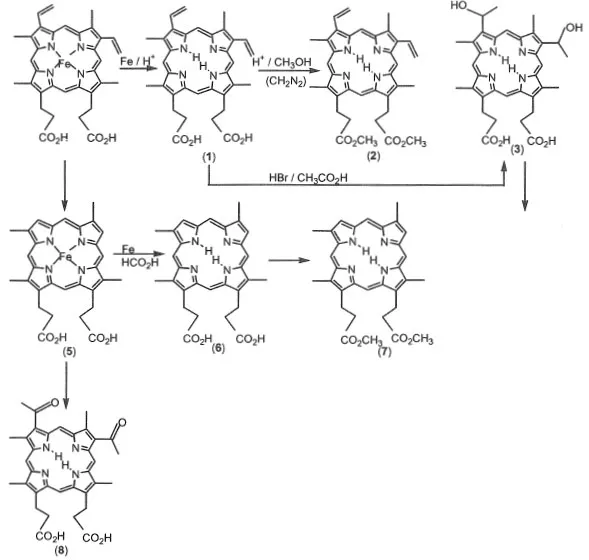
Scheme 1-1 Synthesis of porphyrins from natural heme.
Deuteroporphyrin IX (5) is prepared from protohemin as the starting material.3) Deuterohemin is produced by refluxing protohemin with resorcinol. Iron ion in deuterohemin is removed by refluxing with iron in formic acid. After demetallation, deuteroporphyrin IX methylester is obtained by esterification of protoporphyrin IX in HCl-methanol solution. 2,4-Diacetyldeuteroporphyrin IX is synthesized by the acylation of deuterohemin treated with acetic anhydride and tin chloride.3)
2,4-Diformyldeuteroporphyrin IX is prepared by the oxidation of protoporphyrin IX dimethylester using KMnO4/MgSO4 as oxidizing reagents in acetone. 2,4-Diformyldeuteroporphyrin IX is insoluble in organic solvents.4)
1.1.2 Synthesis of alkyl-substituted porphyrins
The representative alkyl-substituted porphyrins are etioporphyrin (EtioP) and octaethylporphyrin (OEP).5) There are three synthesis routes of OEP, as shown in Scheme 1-2.
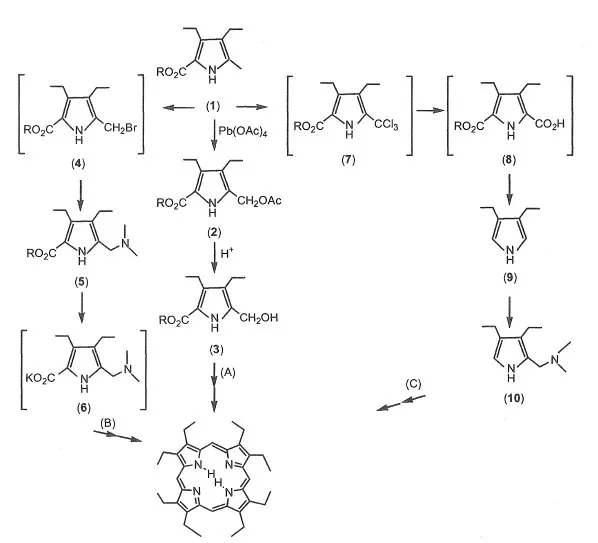
Scheme 1-2 Synthesis routes of OEP.
Route A5): The derivative (2) is synthesized by the acylation of a-methyl of pyrrole derivative (1) using Pb(OAc)4. The derivative (3) is prepared by hydrolysis of (2) in alkaline solution. OEP is synthesized by cyclization and oxidation of (3). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystallization from chloroform and methanol.
Route B6): The derivative (4) is synthesized by bromination of (1). The diethylamino-methyl derivative (5) is synthesized by nucleophilic substitution of amine to (4). The potassium salt (6) is obtained by hydrolysis of (5). OEP is synthesized by cyclization and oxidation of (6). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystal-lization from chloroform and methanol.
Route C7): 3,4-Diethylpyrrol (9) is prepared by decarboxylation of (8). The derivative (10) is synthesized by aminomethylation of (9) in formaldehyde and dimethylformamide. OEP is prepared by cyclization and oxidation of (10). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystallization from chloroform and methanol.
Octamethylporphyrin (OMP) is synthesized using the above methods.8) The yield of OMP is very low and OMP is insoluble in organic solvent.
1.1.3 Synthesis of meso-tetraphenylporphyrins
Rothemund synthesized meso-tetraphenylporphyrin(TPP) by condensation between pyrrole and benzaldehyde.9) Since this report, meso-tetraphenylporphyrin derivatives are synthesized using benzaldehyde derivatives. The yield of porphyrin improves especially when propionic acid is used as a solvent. Porphyrins are usually purified using column chromatography (silica gel or almina).10) The synthesis route is shown in Scheme 1-3. Tetranitrophenylporphyrin, tetratolylporphyrin, tetra(4-pyridyl) porphyrin and tetra(pentafluorophenyl)porphyrin are synthesized by this method.
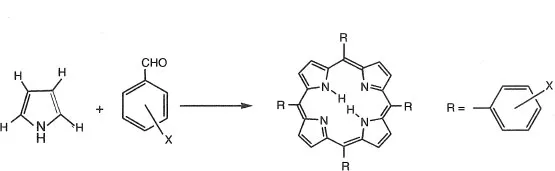
Scheme 1-3 Synthesis routes of meso-tetraphenylporphyrin derivatives.
1.1.4 Synthesis of water-soluble porphyrins
Water-soluble porphyrins, tetraphenylporphyrin tetrasulfonate (TPPS), is synthesized by the sulfonation of TPP using sulfuric acid. Tetrakis-(aminophenyl)porphyrin (TAPP) is synthesized by the reduction of tetrakis(4-nitrophenyl) porphyrin under Sn / HCl condition. Tetrakis-(4-carboxyphenyl)porphyrin (TCPP) is synthesized by hydrolysis of tetrakis-(4-methylcarboxyphenyl)-porphyrin in alkaline alcohol solution. Tetrakis-(4-methylpyridyl)porphyrin (TMPyP) is prepared by quaterization of tetra(4-pyridyl)porphyrin with methyliodide or p-toluenesulfonate methylester in DMF.
1.1.5 Synthesis of metalloporphyrins
Metalloporphyrins are widely use...