
eBook - ePub
Photosensitization of Porphyrins and Phthalocyanines
Ichiro Okura
This is a test
Buch teilen
- 252 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Photosensitization of Porphyrins and Phthalocyanines
Ichiro Okura
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
Photosensitization of Porphyrins and Phthalocyanines covers the scentific background to porphyrins and phthalocyanines, and applications of the compounds, especially for the application for photosensitization. It also has a review of advances in research and applications in this field.
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Photosensitization of Porphyrins and Phthalocyanines als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Photosensitization of Porphyrins and Phthalocyanines von Ichiro Okura im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Medicina & Biochimica in medicina. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
Part I
Synthesis and Characterization of Porphyrins and Phthalocyanines
Chapter 1 Preparation of Porphyrins and Phthalocyanines
1.1 General Methods for Preparation of Porphyrins
Porphyrins are classified into three categories: (A) porphyrins prepared from natural heme, (B) alkyl-substituted porphyrins and (C) meso-tetraphenylporphyrin and its derivatives. In this section, the preparation methods of the representative porphyrins in each category are described. The preparation methods of water-soluble porphyrins (D), metalloporphyrins (E) and chlorin derivatives (F) are also provided.
1.1.1 Synthesis of porphyrins from natural heme (Scheme 1-1)
Protoporphyrin IX (1) is prepared from protohemin as a starting material. Iron ion in the protohemin is removed by refluxing with iron in formic acid. After demetallation, protoporphyrin IX is obtained by the addition of ammonium acetate. Protoporphyrin IX methylester (2) is synthesized by esterification of protoporphyrin IX in HCl-methanol solution.1)
Hematoporphyrin IX (3) is prepared as follows. Protoporphyrin IX is treated with HBr-acetic acid to convert the vinyl group to a hydroxyl group and is then neutralized by NaOH. By the addition of excess NaOH solution, hematoporphyrin IX is obtained as a precipitate.
meso-Porphyrin IX (4) dimethylester is prepared as follows.2) The vinyl group of hematoporphyrin IX dimethylester is reduced to an ethyl group by hydrogen gas catalyzed by palladium-active carbon under methylmethacryrate and formic acid. meso-Porphyrin IX dimethylester is easily recrystallized from chloroform-methanol. This compound is most popular among the porphyrins from natural heme.
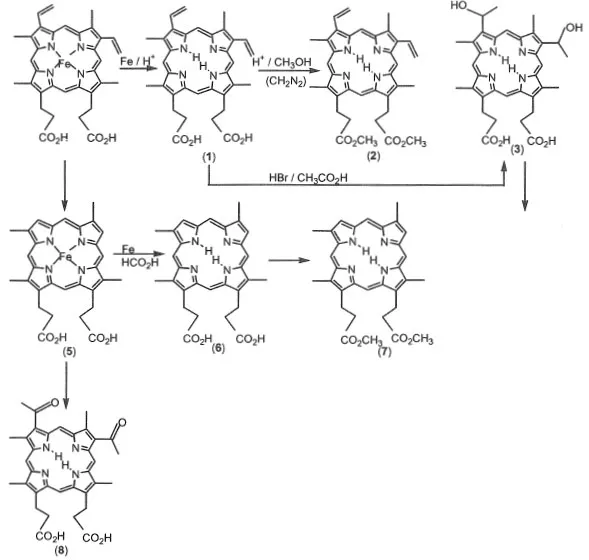
Scheme 1-1 Synthesis of porphyrins from natural heme.
Deuteroporphyrin IX (5) is prepared from protohemin as the starting material.3) Deuterohemin is produced by refluxing protohemin with resorcinol. Iron ion in deuterohemin is removed by refluxing with iron in formic acid. After demetallation, deuteroporphyrin IX methylester is obtained by esterification of protoporphyrin IX in HCl-methanol solution. 2,4-Diacetyldeuteroporphyrin IX is synthesized by the acylation of deuterohemin treated with acetic anhydride and tin chloride.3)
2,4-Diformyldeuteroporphyrin IX is prepared by the oxidation of protoporphyrin IX dimethylester using KMnO4/MgSO4 as oxidizing reagents in acetone. 2,4-Diformyldeuteroporphyrin IX is insoluble in organic solvents.4)
1.1.2 Synthesis of alkyl-substituted porphyrins
The representative alkyl-substituted porphyrins are etioporphyrin (EtioP) and octaethylporphyrin (OEP).5) There are three synthesis routes of OEP, as shown in Scheme 1-2.
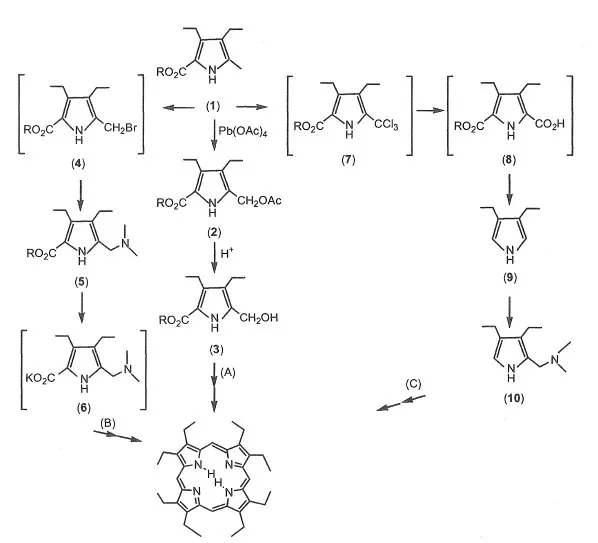
Scheme 1-2 Synthesis routes of OEP.
Route A5): The derivative (2) is synthesized by the acylation of a-methyl of pyrrole derivative (1) using Pb(OAc)4. The derivative (3) is prepared by hydrolysis of (2) in alkaline solution. OEP is synthesized by cyclization and oxidation of (3). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystallization from chloroform and methanol.
Route B6): The derivative (4) is synthesized by bromination of (1). The diethylamino-methyl derivative (5) is synthesized by nucleophilic substitution of amine to (4). The potassium salt (6) is obtained by hydrolysis of (5). OEP is synthesized by cyclization and oxidation of (6). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystal-lization from chloroform and methanol.
Route C7): 3,4-Diethylpyrrol (9) is prepared by decarboxylation of (8). The derivative (10) is synthesized by aminomethylation of (9) in formaldehyde and dimethylformamide. OEP is prepared by cyclization and oxidation of (10). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystallization from chloroform and methanol.
Octamethylporphyrin (OMP) is synthesized using the above methods.8) The yield of OMP is very low and OMP is insoluble in organic solvent.
1.1.3 Synthesis of meso-tetraphenylporphyrins
Rothemund synthesized meso-tetraphenylporphyrin(TPP) by condensation between pyrrole and benzaldehyde.9) Since this report, meso-tetraphenylporphyrin derivatives are synthesized using benzaldehyde derivatives. The yield of porphyrin improves especially when propionic acid is used as a solvent. Porphyrins are usually purified using column chromatography (silica gel or almina).10) The synthesis route is shown in Scheme 1-3. Tetranitrophenylporphyrin, tetratolylporphyrin, tetra(4-pyridyl) porphyrin and tetra(pentafluorophenyl)porphyrin are synthesized by this method.
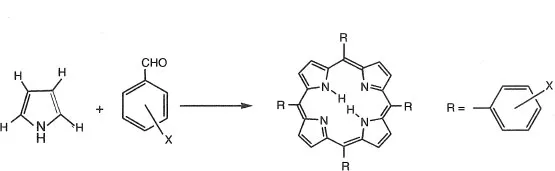
Scheme 1-3 Synthesis routes of meso-tetraphenylporphyrin derivatives.
1.1.4 Synthesis of water-soluble porphyrins
Water-soluble porphyrins, tetraphenylporphyrin tetrasulfonate (TPPS), is synthesized by the sulfonation of TPP using sulfuric acid. Tetrakis-(aminophenyl)porphyrin (TAPP) is synthesized by the reduction of tetrakis(4-nitrophenyl) porphyrin under Sn / HCl condition. Tetrakis-(4-carboxyphenyl)porphyrin (TCPP) is synthesized by hydrolysis of tetrakis-(4-methylcarboxyphenyl)-porphyrin in alkaline alcohol solution. Tetrakis-(4-methylpyridyl)porphyrin (TMPyP) is prepared by quaterization of tetra(4-pyridyl)porphyrin with methyliodide or p-toluenesulfonate methylester in DMF.
1.1.5 Synthesis of metalloporphyrins
Metalloporphyrins are widely use...