
eBook - ePub
Photosensitization of Porphyrins and Phthalocyanines
Ichiro Okura
This is a test
Condividi libro
- 252 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Photosensitization of Porphyrins and Phthalocyanines
Ichiro Okura
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
Photosensitization of Porphyrins and Phthalocyanines covers the scentific background to porphyrins and phthalocyanines, and applications of the compounds, especially for the application for photosensitization. It also has a review of advances in research and applications in this field.
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Photosensitization of Porphyrins and Phthalocyanines è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Photosensitization of Porphyrins and Phthalocyanines di Ichiro Okura in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Medicina e Biochimica in medicina. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
Part I
Synthesis and Characterization of Porphyrins and Phthalocyanines
Chapter 1 Preparation of Porphyrins and Phthalocyanines
1.1 General Methods for Preparation of Porphyrins
Porphyrins are classified into three categories: (A) porphyrins prepared from natural heme, (B) alkyl-substituted porphyrins and (C) meso-tetraphenylporphyrin and its derivatives. In this section, the preparation methods of the representative porphyrins in each category are described. The preparation methods of water-soluble porphyrins (D), metalloporphyrins (E) and chlorin derivatives (F) are also provided.
1.1.1 Synthesis of porphyrins from natural heme (Scheme 1-1)
Protoporphyrin IX (1) is prepared from protohemin as a starting material. Iron ion in the protohemin is removed by refluxing with iron in formic acid. After demetallation, protoporphyrin IX is obtained by the addition of ammonium acetate. Protoporphyrin IX methylester (2) is synthesized by esterification of protoporphyrin IX in HCl-methanol solution.1)
Hematoporphyrin IX (3) is prepared as follows. Protoporphyrin IX is treated with HBr-acetic acid to convert the vinyl group to a hydroxyl group and is then neutralized by NaOH. By the addition of excess NaOH solution, hematoporphyrin IX is obtained as a precipitate.
meso-Porphyrin IX (4) dimethylester is prepared as follows.2) The vinyl group of hematoporphyrin IX dimethylester is reduced to an ethyl group by hydrogen gas catalyzed by palladium-active carbon under methylmethacryrate and formic acid. meso-Porphyrin IX dimethylester is easily recrystallized from chloroform-methanol. This compound is most popular among the porphyrins from natural heme.
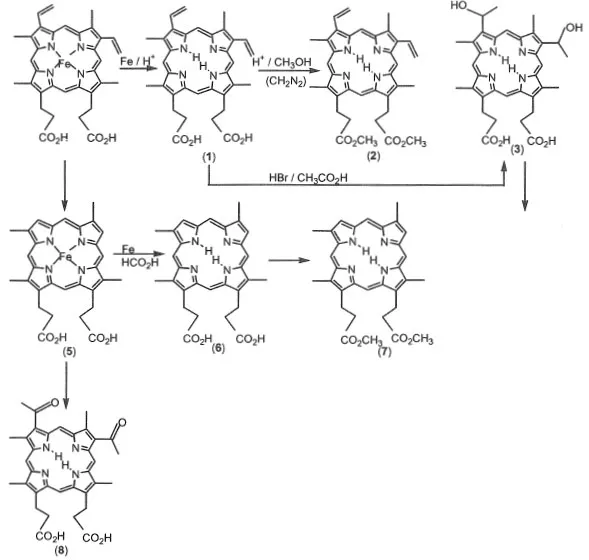
Scheme 1-1 Synthesis of porphyrins from natural heme.
Deuteroporphyrin IX (5) is prepared from protohemin as the starting material.3) Deuterohemin is produced by refluxing protohemin with resorcinol. Iron ion in deuterohemin is removed by refluxing with iron in formic acid. After demetallation, deuteroporphyrin IX methylester is obtained by esterification of protoporphyrin IX in HCl-methanol solution. 2,4-Diacetyldeuteroporphyrin IX is synthesized by the acylation of deuterohemin treated with acetic anhydride and tin chloride.3)
2,4-Diformyldeuteroporphyrin IX is prepared by the oxidation of protoporphyrin IX dimethylester using KMnO4/MgSO4 as oxidizing reagents in acetone. 2,4-Diformyldeuteroporphyrin IX is insoluble in organic solvents.4)
1.1.2 Synthesis of alkyl-substituted porphyrins
The representative alkyl-substituted porphyrins are etioporphyrin (EtioP) and octaethylporphyrin (OEP).5) There are three synthesis routes of OEP, as shown in Scheme 1-2.
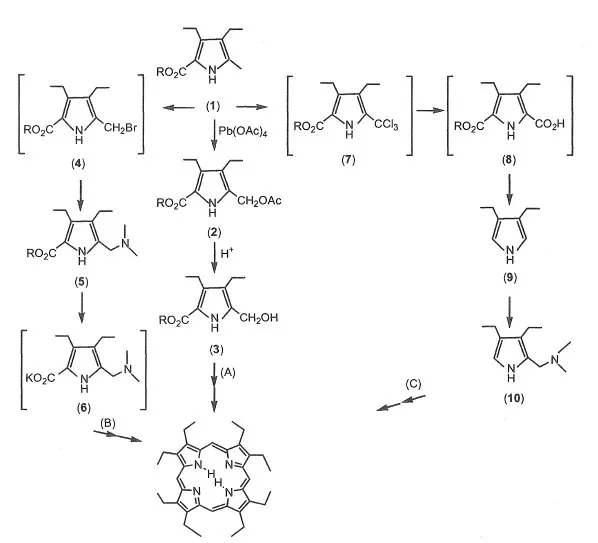
Scheme 1-2 Synthesis routes of OEP.
Route A5): The derivative (2) is synthesized by the acylation of a-methyl of pyrrole derivative (1) using Pb(OAc)4. The derivative (3) is prepared by hydrolysis of (2) in alkaline solution. OEP is synthesized by cyclization and oxidation of (3). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystallization from chloroform and methanol.
Route B6): The derivative (4) is synthesized by bromination of (1). The diethylamino-methyl derivative (5) is synthesized by nucleophilic substitution of amine to (4). The potassium salt (6) is obtained by hydrolysis of (5). OEP is synthesized by cyclization and oxidation of (6). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystal-lization from chloroform and methanol.
Route C7): 3,4-Diethylpyrrol (9) is prepared by decarboxylation of (8). The derivative (10) is synthesized by aminomethylation of (9) in formaldehyde and dimethylformamide. OEP is prepared by cyclization and oxidation of (10). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystallization from chloroform and methanol.
Octamethylporphyrin (OMP) is synthesized using the above methods.8) The yield of OMP is very low and OMP is insoluble in organic solvent.
1.1.3 Synthesis of meso-tetraphenylporphyrins
Rothemund synthesized meso-tetraphenylporphyrin(TPP) by condensation between pyrrole and benzaldehyde.9) Since this report, meso-tetraphenylporphyrin derivatives are synthesized using benzaldehyde derivatives. The yield of porphyrin improves especially when propionic acid is used as a solvent. Porphyrins are usually purified using column chromatography (silica gel or almina).10) The synthesis route is shown in Scheme 1-3. Tetranitrophenylporphyrin, tetratolylporphyrin, tetra(4-pyridyl) porphyrin and tetra(pentafluorophenyl)porphyrin are synthesized by this method.
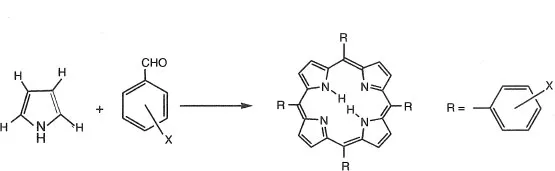
Scheme 1-3 Synthesis routes of meso-tetraphenylporphyrin derivatives.
1.1.4 Synthesis of water-soluble porphyrins
Water-soluble porphyrins, tetraphenylporphyrin tetrasulfonate (TPPS), is synthesized by the sulfonation of TPP using sulfuric acid. Tetrakis-(aminophenyl)porphyrin (TAPP) is synthesized by the reduction of tetrakis(4-nitrophenyl) porphyrin under Sn / HCl condition. Tetrakis-(4-carboxyphenyl)porphyrin (TCPP) is synthesized by hydrolysis of tetrakis-(4-methylcarboxyphenyl)-porphyrin in alkaline alcohol solution. Tetrakis-(4-methylpyridyl)porphyrin (TMPyP) is prepared by quaterization of tetra(4-pyridyl)porphyrin with methyliodide or p-toluenesulfonate methylester in DMF.
1.1.5 Synthesis of metalloporphyrins
Metalloporphyrins are widely use...