
eBook - ePub
Synthetic Applications
Rajender S. Varma, Bubun Banerjee, Rajender S. Varma, Bubun Banerjee
This is a test
Partager le livre
- 454 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Synthetic Applications
Rajender S. Varma, Bubun Banerjee, Rajender S. Varma, Bubun Banerjee
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Magnetic nanocatalysts are becoming an important tool for greener catalytic processes in chemical transformations in view of the ease of their removal from a reaction medium. This book explores assorted magnetic nanocatalysts, their deployment in synthesis, chemical transformation and their recovery and reuse. Various thematic topics embodied include magnetic nanocatalysts for S-S bond formation, N-heterocycle formation, C-heteroatom bond formation, silica-supported catalysts, multicomponent reactions, including their recyclability; another available volume emphasizes the utility of magnetic nanocatalysts in industrial appliances.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Synthetic Applications est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Synthetic Applications par Rajender S. Varma, Bubun Banerjee, Rajender S. Varma, Bubun Banerjee en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Sciences physiques et Nanotechnologie. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
Chapter 1 Magnetic metal nanoparticle-catalyzed carbon-heteroatom bond formation and synthesis of related heterocycles
Brindaban C. Ranu *
Laksmikanta Adak
Indian Institute of Engineering Science and Technology, Howrah, India
Nirmalya Mukherjee
School of Chemical Sciences, Indian Association for the Cultivation of Science, Kolkata, Kolkata, India
Tubai Ghosh
Jadavpur University, Department of Chemistry, Kolkata, India
Acknowledgments: B. C. Ranu gratefully acknowledges the support of the Indian National Science Academy, New Delhi for offering him the position of INSA Honorary Scientist. L. Adak thanks SERB, DST, Government of India (Project: SRG/2020/001350) and the WBDST-BT for their support via government order [Memo No: 1854(Sanc.)/ST/P/S&T/15G-7/2019]. T. Ghosh thanks the UGC-DSKPDF (UGC Award Letter No. & Date: F.4-2/2006 (BSR)/CH/19-20/0088; 24.01.2020) for his postdoctoral fellowship.
1.1 Introduction
The past decade saw an exponential growth in the area of nanoscience and nanotechnology. One of the most interesting features of nanotechnology is its useful applications in various fields. The discovery and easy accessibility of nanoparticles (NPs) of different shapes, sizes, and compositions have prompted investigations on their applications in catalysis. The “nano” in nanocatalysis refers to the size of the particles in the nanoscale range. As nanoparticles have a high surface-to-volume ratio, compared to bulk materials, they offer a viable alternative to conventional catalysts [1, 2]. Recent reports have showed their remarkable performance as catalysts, in terms of reactivity, selectivity, and product yields [3, 4, 5, 6, 7, 8]. Nanocatalysts offer numerous advantages over convention catalyst systems, but isolation and recovery of these small nanocatalysts from the reaction mixture is tedious. The development of an efficient, and industrially and environmentally acceptable catalyst constitutes one of the important goals towards sustainability and economic growth. To achieve this goal, the use of magnetic nanoparticles has received much interest as they are easily separable and reusable. Thus, magnetic nanoparticles have appeared as a practical alternative. Their magnetic and insoluble properties offer easy and efficient separation of the catalyst from the reaction mixture – by the application of an external magnet without the requirement of filtration, centrifugation, or other complex workup.
The carbon-heteroatom bond formation constitutes the backbone of the synthesis of heterocycles. Thus, the carbon-heteroatom bond formation at a high efficiency is of great interest. The formation of the transition metal catalyzed carbon-heteroatom bond by cross-coupling is an efficient tool and has received wide application in the synthesis of pharmaceutically active heterocyclic compounds, drugs, and natural products [9, 10, 11, 12, 13, 14, 15]. In general, as the use of metal nanoparticles provides more advantages over parent metals in catalysis, more investigations are now directed towards the application of magnetic nanoparticles for various fundamental reactions [16, 17, 18, 19, 20, 21, 22]. This chapter will highlight the applications of magnetic metal nanoparticles in the carbon-heteroatom bond formation and the related synthesis of heterocycles.
1.2 Carbon-nitrogen bond formation and the synthesis of related heterocycles
Carbon-nitrogen (C–N) bond formation is one of the useful processes in organic synthesis, as the consequent amines are broadly used as intermediates or precursors in the preparation of fine chemicals, pharmaceuticals, agrochemicals, and natural products. The developments in the formation of carbon-nitrogen bonds and in the synthesis of related heterocycles by magnetic metal nanoparticles as catalysts have been described below.
1.2.1 C–N bond formation via cross-coupling reactions
Martínez et al. [23] reported the reaction of aromatic amines with substituted benzylic alcohols to produce the respective benzyl imines, in the presence of magnetite (Figure 1.1). The reaction involved 20 mol% of magnetite (Fe3O4) and two equivalents of t-BuOK (potassium t-butoxide) as a base in 1,4-dioxane at 90 °C for a period of 7 days. Diversely substituted anilines (1) reacted with various benzyl alcohols (2) under standardized reaction conditions and the corresponding N-substituted benzylated derivatives (3) were obtained at excellent yields. Benzylic alcohols were used as electrophiles here. The best result was observed when less nucleophilic 3-chloroaniline was used, and the use of more nucleophilic aniline derivatives led to lower yields of the corresponding products. No reaction was observed with aliphatic amine or aliphatic alcohols, which clearly indicates the preference of the catalyst. Using a simple external magnet, the catalyst was recovered and reused eight times without any loss of significant catalytic activity.
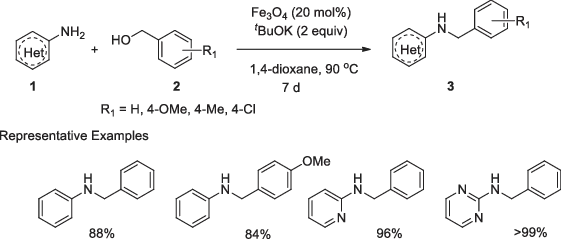
Figure 1.1: Fe3O4-catalyzed N-monoalkylation of aromatic...