About this book
Answers at your fingertips to the essentials of a subject that is challenging at best and that many students struggle with. The 6 page laminated guide focuses on physical chemistry with the details and structure of the subject organized and designed to be a key to the answers that are further supported by your texts and lectures. Use as a review before testing, or as a memory companion that keeps your mind focused on the whole course daily, weekly, or as needed before exams. Suggested uses:
o Students – especially relevant for those majoring in engineering, science, or a health care related field
o Quick Reference – instead of digging into the textbook to find a core answer you need while studying, use the guide to reinforce quickly and repeatedly
o Memory – refreshing your memory repeatedly is a foundation of studying, have the core answers handy so you can focus on understanding the concepts
o Test Prep – no student should be cramming, but if you are, there is no better tool for that final review
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information

- Acids produce H+ ions in aqueous solutions HCl (aq) → H+ (aq) + Cl- (aq)
- H+ ions react with water to form H3O+ (hydronium) ions
- Bases produce OH- ions in aqueous solutions NaOH (aq) → Na+ (aq) + OH- (aq)
- Acids and bases form water and neutralize each other H+ (aq) + OH- (aq) → H2O (l)
- Acids are proton (H+) donors HCl (aq) + H2O (l) → H3O+ (aq) + Cl- (aq)
- HCl is an acid because it donated a H+ to H2O
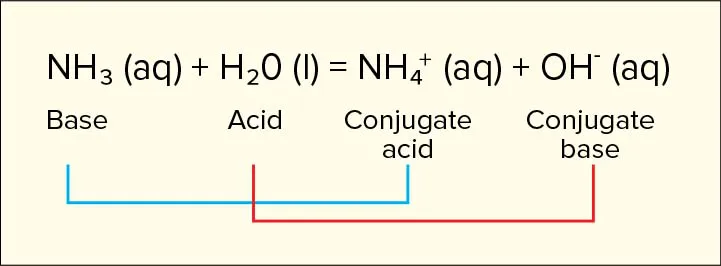
- Bases are proton (H+) acceptors NH3 (aq) + H2O (l) → NH4+ (aq) + OH- (aq)
- NH3 is a base because it accepted the H+ from H2O
- Conjugate acid-base pairs: Transfer of a proton H2SO4 (aq) + H2O (l) → HSO4- (aq) + H3O+ (aq)
- Transfer of electron pairs
- Acids are electron pair acceptors
- Bases are electron pair donors
- A substance doesn’t need to contain hydrogen to be an acid

- BF3 has an empty orbital and can accept an electron pair from NH3
- The product of a Lewis acid-base reaction is called an adduct
- The Lewis definition creates a new class of acids
- Lewis acids have empty orbitals
- Molecules with incomplete octets act as Lewis acids
- Molecules with complete octets act as Lewis acids and may rearrange electrons
- Some cations act as Lewis acids and have empty octets
- Strong acids completely ionize in a solution HCl (aq) + H2O (l) → H3O+ (aq) + Cl- (aq)
- A 1.0 M HCl solution has [H3O+] of 1.0 M
- Weak acids partially ionize in a solution HF (aq) + H2O (l) ↔ H3O+ (aq) + F- (aq)
- A 1.0 M solution HF has [H3O+] less than 1.0 M
- General formula: HA (aq) + H2O (l) ↔ H3O+ (aq) + A- (aq)HA (aq) ↔ H+ (aq) + A- (aq)
- Autoionization: Water acts as an acid and a base H2O (l) ↔ H+ (aq) + OH- (aq)Kw = [H3O+][OH-] = [H+][OH-] = 1.0×10-14
- Neutral solution: [H3O+] = [OH-] = 1.0×10-7 M at 25°C
- Acidic solution: [H3O+] > [OH-]
- Basic solution: [H3O+] < [OH-]
- pH: Measure of the acidity of a solution pH = -log [H3O+]
- pH > 7: Basic solution
- pH < 7: Acidic solution
- pH = 7: Neutral solution
- pOH: Uses [OH-] pOH = -log [OH-]
- pOH < 7: Basic solution
- pOH > 7: Acidic solution
- pOH = 7: Neutral solution
- pH + pOH = 14.00 at 25°C
- pKa = -log Ka
- Small Ka: Strong acid
- The relative strength of weak acids is determined by the acid ionization constant
[H3O+][A−] |
[HA] |
- ICE charts: Compare initial concentrations, changes, and concentrations at equilibrium HA (aq) + H2O (l) ↔ H3O+ (aq) + A- (aq), where: [HA] is 0.10 M
- The initial [H3O+] is roughly 0.00—a small amount from the autoionization of water
[H3O+][A−] |
[HA] |
x2 |
0.10 − x |
-
-
- x is the amount of HA ionized
- x is usually very small and can be ignored in most circumstances
- If x isn’t very small, use the quadratic equation to solve
-
[HA] | [H3O+] | [A-] | |
| Initial | 0.10 | ~0.00 | 0.00 |
| Change | -x | +x | +x |
| Equilibrium | 0.10 − x | x | x |
- Percent ionization: Weak acids; percent of the initial acid that ionizes
- Percent ionization =
× 100%(Concentration of ionized acid)(Initial concentration of acid)
- Percent ionization =
- Strong bases completely dissociate
- NaOH (aq) → Na+ (aq) + OH- (aq)
- Most strong bases are in group 1A or 2A of the periodic table
- Weak bases don’t completely dissociate NH3 (aq) + H2O (l) ↔ NH4+ (aq) + OH- (aq)
- Base ionization constant: Kb
- Generic formula: B (aq) + H2O (l) ↔ BH+ (aq) + OH- (aq)Kb =[BH+][OH-][B]Ka× Kb = Kw = 1.0 × 0-14 at 25°CpKa + pKb = 14
- Salt: Result of the neutralization of an acid and base; ionic compound
- Has both a cation and an anion
- Can form acids, bases, or neutral solutions in water
- When the cation and anion are both neutral, it forms a pH neutral solution in water
- When the cation is neutral and the anions are a conjugate base of weak acid, it forms a basic soluti...
Table of contents
- Intermolecular Forces
- Changes in Matter
- Solutions
- Chemical Kinetics
- Acids & Bases
- Thermodynamics
- Electrochemistry
- Radioactivity
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
